Radiological Assessment of Recent Sediments In Qua Iboe River South-South Nigeria
Corresponding Author
Email: obongabasiorok@gmail.com
Affiliation
Osabor V. N., Emmanuel E Duke Okon* and Ekpechi D.C
Department of Pure and Applied Chemistry University of Calabar-Nigeria
Article Reviewed By:
Zeynep Aydogan(zeybionep@gmail.com)
Umit Duru(umitduru@sakarya.edu.tr)
Hussein Kanbar(hussein.kanbar@univ-lorraine.fr)
Vera I Slaveykova(Vera.Slaveykova@unige.ch)
Citation
Emmanuel E Duke Okon, Radiological Assessment of Recent Sediments In Qua Iboe River South-South Nigeria(2017)SDRP Journal of Earth Sciences & Environmental Studies 2(3)
Abstract
An assessment of radiological activity levels of Qua Iboe River Sediments was carried out. A total of ten sediment samples were obtained and analysed using a gamma ray spectrometer with a high purity germanium detector. The samples were also subjected to total organic carbon (TOC) content and pH determinations. The result of TOC ranged from 1.60 to 6.50% with a mean value of 4.93%. The pHranged from 4.12 to 4.86 with a mean value of 4.93 was obtained. The activity concentration of 40K ranged from 69.44 to 306.71Bq/Kg with a mean value of 206.02Bq/Kg. 232Th ranged from 12.56 to 26.09Bq/kg with mean value of 18.34Bq/Kg. 238Uranged from 20.58 to 38.61Bq/kg with a mean value of 28.14Bq/Kg while 137Cs values ranged from 1.41 to 1.76Bq/Kg with a mean value of 0.92Bq/Kg. Pearson and Kendall correlation matrices employed on this study showed positive and negative correlation among variables with the sediment. Estimation of radiological hazard was also calculated and the mean result obtained for the representative level index (Iy), external hazard index (Hex) and internal hazard index (Hin) were 0.52, 0.19 and 0.27 respectively. The results obtained from the present study revealed that the sediment is safe for building materials and the aquatic organisms are not exposed to infused radioactive radiation. Keywords: Radiological, Sediments and Qua iboe River.
Introduction
The Qua Iboe River of South-South Nigeria and the organism in the ecosystem are consistently exposed to wide varieties of different ionizing radiation from both natural and artificial radionuclides (El Samad et. al., 2013) Natural radioactivity is widespread in the earth’s surface and it exists in various geological formations such as soil, rocks, water, sediments and in building materials.
A radionuclide or radionuclides are atoms that possess excess nuclear energy that makes them unstable (Petrucci et. al., 2002). Due to the presence of high energy in the nucleus of these particles, excess of this energy can be transferred to one electron causing it to be ejected. This process may lead to radioactive decay (Petrucci et. al., 2002). Radioactivity is a phenomenon in which particles are emitted from the nuclei of an element as a result of nuclear instability. High exposure of radiation to human causes cancers for example hazardous radionuclides like strontium 90and iodine 131 (Ashnani et. al., 2010)
Agbalagba et. al. (2001) measured the natural radioactivity content in soil and sediment concentration in water samples in the flood plain lakes of the Niger Delta. Their findings reveal some levels of natural radionuclides 226Ra, 232Th and 40K in the samples.
Umar et. al. (2012) studied the natural radioactivity in environmental samples (soil, vegetation and water) from the Idu industrial district of federal capital Territory (FCT) Abuja. The results from their work revealed some levels of natural radionuclides.
Olatunde et. al. (2011) measured the radioactivity levels in the coastal areas of Nigeria. The results obtained revealed appreciable levels of radionuclides in the region. In the literature much information have been documented on heavy and trace elements in the environment but information on the assessment of radiological elements is very scanty. The present study was designed to assess the levels of radioactive elements on recent sediments of Qua Iboe river system with a view to predicting their pollution status.
The study Area:
The Qua Iboe River estuary lies on the South-Eastern Nigeria Coastline. It is a meso tidal estuary having tidal amplitude of 1m and 3m at near and spring phases respectively. The river originates from Umuahia hills and transverses mainly through the sedimentary terrains of cretaceous to recent ages and develops into extensive meanders before emptying into the Atlantic Ocean. Creeks and channel islands are common throughout the length of the estuary while sand bars occurs at the mouth as a result of interplay between the long shore drift which runs approximately in a West-East direction of the river current. The area has some coastal plain sands which are not older than the quaternary age. Sediments are brought into the estuary by long shore drift, tide flow, waves and river transport. Coarse to medium grained sand occurs mostly on the mouth of the estuary and the middle of the main channel where the tidal currents are strong. Most part of the bank and creeks are weak and are characterized by fine sand, silt and clay. The latter has a high affinity for pollutants such as hydrocarbons and metals. The climate of the area is characterized by a long wet season usually lasting from April to November and a short period of dry season from December to March. (Ekwere, Akpan and Ntekim, 1992) .
Materials & Methods
Sediment sample collection
Ten (10) sediment samples; five considered bland for preliminary test, were collected from seven locations along the Qua Iboe river estuary using the Van Veen Grab sampler. The samples were placed in polyethylene bags and were placed in a cooler containing ice to maintain the temperature at about 40C and transported within 2 hours to the laboratory for analysis. The sediment samples on reaching the laboratory were air dried, disaggregated on an agate mortar and sieved to 2 mm sizes. Each sample was sealed in cylindrical plastic container. The sealed samples were kept for at least 28 days to attain secular equilibrium before gamma spectrometric analysis commenced.
Particle size analysis
Particle size was determined using the Bouyocous hydrometer method in the same vein as Burmamu and Law 2015. The particle size in percent of each constituent was derived from the expression.
H1 + [0.3(T1-20)-2]
Total organic carbon (TOC) content determination (Modified Walkley-black 1934 method)
Total organic carbon (TOC) content of the samples as well as the blank (control) was determined using the Modified Walkley-black method (Edu et. al. 2013) Percentages total organic carbon in each sample was calculated using the equation below, and results recorded.
%Organ Carbon = 10(1-T/S)x F
Determination of pH
7.0 pH buffer solution was added to a beaker and pH electrode inserted to calibrate the pH meter to a pH accordingly in the same perspective as Avery et. al., 1974.The values were then read off and data obtained recorded
Analysis of radioactive substances
The counting equipment used consists of a Canberra vertical cylindrical high-purity coaxial germanium (HP-Ge) detector model GC2018-7500 and serial number b 87063, enclosed in a 100 mm thick lead shield to reduce noise and impedance. The HP-Ge detector was connected to a Canberra computer-assisted Multichannel analyzer (MCA). Accurate energy and efficiency calibrations of the gamma-spectrometer system were made using standard sources of radionuclides supplied by the International Atomic Energy Agency (IAEA), Vienna, Austria and the Isotope Products Laboratories, Burbank California, USA. The descriptions of the gamma spectrometric system as well as more details on the calibration are well documented (Fasasi et. al., 1999). An empty Marinelli beaker with the same geometry as that of the sample was used as background. The counting time for accumulating spectral analysis for both the samples and background was set at 36,000 s. Each container was counted twice in order to establish precision and accuracy of the counting system; the data of the first count in each case was considered rough reading hence not recorded in the table. The gamma spectroscopic analysis employed in this work was based on a computer program SAMPO 90, The resolution of the HPGe detector made it possible to identify a large number of g–rays in the samples The photopeaks observed with regularity in the samples were identified as belonging to the radioactive decay series of 238U and 232Th and non-series radionuclides 40K and 137Cs. In some samples 137Cs radionuclide appeared at low levels, or occurred at levels below the detectable limit (BDL). The specific radio-activities of 40K and 137Cs were determined directly by their g –lines of energies 1460.8 and 661.3 keV respectively while that of 238U and 232Th were estimated by taking the mean of specific radioactivities obtained from the g –ray lines of energies 609.3 and 1120.3 keV of 214Bi; for 238U and 969.0 keV of 228Ac and 583.0 keV of 208Tl for 232Th.
Results
Sediment bulk properties
The sediment bulk parameters for the four sediments were different and these variations may influence the radioactivity levels in the study area. Values of pH ranged from 4.12-4.86 (Table 1) with a mean value of 4.39±0.33. The moderately acidic nature observed in the sediment of this study may be attributed to influence of surface run-off which was confirmed by the predominance of sand fraction of the sediment. However, low total organic carbon (TOC) were recorded in this study ranging from 1.60 to 6.50 % maximizing at SD2 and minimizing at SD5 with a mean value of 3.93±0.81 % (Table 1). The prevalence of low TOC content in the study area might be a result of poor absorbability of the OC on the solid matrix, characteristic sheltered morphology of the basin predominated by sand fraction and the mixing process at the sediment-water interface; at that, the rate of delivery and the rate of degradation by microbial-mediated processes can be high.
Concentration of radioactive substances
The concentration of the radionuclides observed in the surface sediment samples of this study is presented in table 1 above. The activity concentration of 40K ranged from 69.44 to 306.71 Bq/kg with a mean value of 206.02±91.6 Bq/kg maximizing at SD5 and minimizing at SD3 (Table1). The high activity concentration of 40K at SD5 may be attributed to tidal influence between the Atlantic Ocean and the study area since potassium is a major element in the ocean. However, considering 40K activity concentration as high as 6500 Bq/kg have been reported in phosphate fertilizers (Khater and Al-sewaidan, 2008), their extensive use by farmers may be a significant source of 40K radionuclide in the Qua Iboe River. 137Cs activity concentration in the present study ranged from 1.41 to 1.76 Bq/kg with a mean value of 1.54±0.19 Bq/kg (Table 1). The maximum value was recorded at SD3 while the minimum value was recorded at SD4 meanwhile 137Cs was below detection level at SD2 and SD5. The low activity concentration of 137Cs as well as its non-detection in the surface sediment samples in the present study may be linked to the absence of atmospheric fall-out, weapon testing and land-based nuclear installation within the study area. This is in line with the work of Mackenzie et. al., (1998) that though 137Cs has been deposited throughout the world, higher concentrations are seen in the Northern Hemisphere than in the Southern Hemisphere as a result of nuclear weapon testing. The activity concentrations of 232Th were found ranging from 12.56 to 26.09 Bq/kg with a mean value of 18.34±5.11 Bq/kg (Table 1). The highest value was recorded at SD3 while the lowest value was recorded at SD4. Thorium is naturally present in the soil, rocks, surface water, groundwater, plants, and animals at low concentrations, in the order of ten parts per million. Higher levels are present in certain geological materials such as monzanite sands. Essentially all naturally occurring thorium is present as thorium-232. Thorium preferentially adsorbs firmly to soil particles, with concentrations in sandy soil generally more than 3,000 times higher than in interstitial water, it is even less mobile in clay soils, with concentration ratios over 5,000 (Human Health Fact Sheet, 2001). Therefore, the low value of 232Th in the present study may be attributed to the predominance of coarse sand fraction of the sediment. More so, 238U values were found ranging from 20.58 to 38.21 Bq/kg with a mean value of 29.15±7.55 Bq/kg (Table 4). The highest and lowest value was recorded at SD2 and SD4 respectively.
Radiological correlation matrices
The relative radiological correlation between the levels of 40K, 137Cs, 232Th, 238U, pH, TOC, Sand, Silt and Clay in the sediment samples in this study were tested separately with Pearson and Kendall correlation matrices and the indices obtained are as presented in Tables 2 and 3 respectively. Pearson correlation (Table 2) reveals positive significance correlation index occurring between 232Th and 238U (0.649), 232Th and TOC (0.614), 238U and TOC (0.826), 232Th and silt (0.626). From the Pearson correlation matrices index we posit that particle size and TOC present the determinant factors of the deposition of 232Th and 238U in sediment of Qua Iboe River. In the same vein, it is observed that increase in 232Th correlation index points to resultant increase in 238U content.
On the other hand, Kendall correlation points to relative increase in both 232Th and 238U content in the sediment as observed in the positive significance of 0.6. Kendall correlation index also strongly points to TOC as the controlling factor of 238U with a significance correlation of 0.8 as can be observed in Table 3 below.
Estimation of the radiological hazard indices
Table 4 presents some radiological effects indices such as the Representative Level index, External Hazard Index (Hext), and Internal Hazard Index (Hin).
Representative level index (Iγ)
Radiation hazard index also referred to as representative levels index Iγ is given by (See Equation1 in Figures & tables below)
The Representative Level Index ranged between 0.38 - 0.58 with an average of 0.52 (Table 4). The lowest and the highest values were obtained in station SD4 and SD2. It should be noted that all Iγ values observed are within a very narrow range and are less than unity, the upper limit for the representative level index. These confirm that the sediment investigated exhibit very low gamma-radiation levels.
Internal hazard index (Hin)
The internal hazard index (Hin) is given as
Hin = Cu/185 + CTh/259 + Ck/4810 (Beretka and Mathew, 1985).
Hin should be less than unity for the radiation hazard to be negligible. Hin ranged between 0.20 and 0.32 Bq/kg from station SD4 and SD2 with a mean of 0.27 Bq/kg as contained in Table 4. The highest value of Hin is still below the limit of 1 Bq/kg (Iqbal et. al., 2000) for the safe use of the sediment in the construction of houses.
External hazard index
Many radionuclides occur naturally in soils and rocks upon detritions which then wash to River beds, these radionuclides produce external radiation fields to which all aquatic organisms are exposed. In terms of dose, the principal primordial radionuclides are 232Th, 238U and 40K. Thorium and Uranium are at the topmost of the series of radionuclides that produce significant organism’s exposure. The external hazard index (Hex) enables us to analyse the radiation dose expected to be derived externally in a building using these materials (Beretka and Mathew, 1985).
Hex = Cu/370 +CTh/259 +Ck/4810
Where Cu, CTh and Ckare the radioactivity concentrations in Bqkg-1 of 238U, 232Th and 40K respectively. Hin should be less than unity for the radiation hazard to be negligible. The results shown in table 4 revealed that the external hazard index Hex ranged between 0.14 and 0.22 Bq/kg from SD4 and SD2 respectively, with a mean of 0.19 Bq/kg. The overall result is still below the limit of 1 Bq/kg. Hence the sediment is safe to be used as building materials.
Conclusion
Radiological indices of selected radioactive substances in recent sediment samples of Qua iboe River South-South Nigeria, were investigated. From the results obtained, the sediment samples revealed high levels of activity of 40K, 232Th, 235U and 132Cs.
Radiological hazard indices were also calculated and the mean value for the representative index; external hazard and internal hazard index were less than unity (0.52, 0.19 and 0.27 Bq/Kg). these values fall within the world range for sediment as building materials. The major controlling factor of the activity concentration of the radionuclide in the study area was the sediment grain size. Pearson and Kendall correlation analyses revealed positive correlation.
Images and Tables
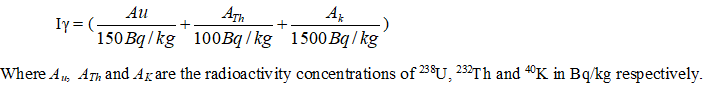
References
Agbalagba, E.O., Avwiri, G.O. and Chad-Umoreh, Y.E.(2012). "y-spectroscopy measurement of natural radioactivity and assessment of radiation hazard indices in soil samples from oil fields environment of Delta state Nigeria". Journal of Environmental Radioactivity, 109: 4-70. PMid:22310017
View Article PubMed/NCBIAshnani, M.H., Yavari, A.R. and Hassani, E. (2010). A survey of pollutions of the Aras River and the Southwest of the Caspian Sea case study. Radioactivity Pollutions. World Applied Sciences Journal, 9 (1): 76-80.
Avery, B.W. and Bascomb, C.L. (1974). Soil survey laboratory methods technology monogramme no. 6. Soil Survey Rothmsted Exp. Stn. Harpenden.
Burmamu, B. R. and Law, P. L. (2015) Evaluation of Some Physical Properties of Soils as Factors Influencing Movement of Contaminants through Porous Soils Media of Gwari Market Dumpsites, Yola, Nigeria. International Research Journal of Engineering and Technology 2(9): 389 ? 397.
Beretka, J. and Mathew, P.J. (1985). Natural radioactivity of Australia Building materials industrial wastes and by-products, Health Physics. PMid:3967976
View Article PubMed/NCBIEdu, Elena-Mihaela, Mihalache, M. and Ionescu, M. (2013). Determination of Organic Carbon in Forest Soils by Comparative Analysis of Methods: Walkley Black method with the Gogoasa modification versus Dry combustion Dumars method. Research Journal of Agricultural Science 45(1): 13 ? 20.
Ekwere, S.J., Akpan, E.B. and Ntekim, E.E.U. (1992). Geochemical studies of Qua Iboe River sediments. Journal of Tropical Sciences, 2:91-100.
El Samad, O., Baydoun, R., Nsouli, B., and Darwish, T. (2013). Determination of natural and artificial radioactivity in soil at North Lebanon province. Journal of environmental radioactivity, 125, 36-39. PMid:23498968
View Article PubMed/NCBIElegba, S. (1993). Uses of radioactive sources in the petroleum industry. Proceedings of Workshop on Radiation Safety in the Nigerian Petroleum Industry, June 23-25 Lagos, Nigeria.
Fasasi, M.K., Tchokossa, P., Ojo, J.O. and Balogun, F.A. (1999). "Occurrence of natural radionuclide and fallout cesium-137 in dry season agricultural land in south western Nigeria". Journal of Radiation and Analytical and Nuclear Chemistry, 240(3):949.
View ArticleFrederick, S. (1999). "The radio elements and the periodic law". Chem. News, Nr. 107:97-99.
Human Health Fact Sheet (2001). Retrieved No. 3 2015.
ICRP, 1979. Limits for Intakes of Radionuclides by Workers. ICRP Publication 30 (Part 1). Ann. ICRP 2 (3-4).
Iqbal, M., Tufail, M. And Mirza, S.M. (2000). Journal of Environmental Radioactivity, 51(2):255-265. 00077-1
View ArticleIvanovich, M and Harmon, R. S. (1992). "uranium-seriesdisequlibrium application to earth marine and environmental sciences'' 2nd edition oxford clarendon press,
Jagdish, K. (2005). "Nuclear wallet cards". 7th edition. April, 2005.
Jibri, P. and Okeyode, O. (2012). Evaluation of radiological hazards in the sediments of Ogun River
View ArticleKhater and Al-Sewaidan (2008). Radiation exposure due to agricultural uses of phosphate fertilizers. Radiation Measurements, 43:1402-1407.
View ArticleLoveland, W. and Morrissey, D. (2006). Modern Nuclear Chemistry Wiley. Interscience page 57 ISBN 0-471-11532-0.
MacKenzie, A.B., Cook, G.T., McDonald, P. and Jones, S.R. (1998). The influence of mixing timescales and re-dissolution processes on the distribution of radionuclides in Northeast Irish Sea sediments. Journal of Environmental Radioactivity,39(1):35-53. 00044-1
View ArticleMalanca, A., Pessina, V. and Dallara, G. (1993). Assessment of the natural radioactivity in the Brazilian state of Rio-Grande. Health Physics, 65 (3): 298-302. PMid:8244700
View Article PubMed/NCBIMcAulay, I.R. and Moran, D. (1988), Natural radioactivity in soil in republic of Ireland. Radiation protection Dosimetry 24,47-49.
View ArticleMebius, L.J. (1960). A rapid method for the determination of organic carbon in soil, 22:120-124.
Neves, O., Breu, M.M. and Vicente, E.M. (2008). "Uptake of uranium by lettuce (Latucasativa L.) in natural uranium contaminated soils in order to assess chemical risk for consumers". Water, Air and Soil Pollution, 195(4):73-84.
View ArticleNgachin, T. (2007). Assessment of Natural radioactivity and associated radiation hazards in some Cameroonian building material. Radiation Measurements, 42:1861-1867.
View ArticleNorse, D. A.S (2006). Naturally occurring Radionuclides in the marine environment on overview of current knowledge with Emphasis on the North Sea Area 26-48.
Olatunde, M.O., Idowi, P.I. and Ayodeji, O.A. (2011). "Natural radionuclide concentrations and radiological impact assessment of river sediments of the coastal areas of Nigeria". Journal of Environmental Protection, 2:418-423.
View ArticlePentreath, R.J. (1988). Sources of artificial radionuclides in the marine environment in radioactivity and oceanography. Elsevier London Press.
Petrucci, R.H., Harwood, W.S. and Herring, F.G. (2002). General Chemistry p1025-1026.
Ramasamy, V., Suresh, G., Rajkumar, P., Murugesans, S. and Meenakshindaram, V. (2011). Reassessment and comparison of natural radioactivity levels in relation to granulometric contents of recently excavated major river sediments. Journal of Radio Analytical and Nuclear Chemistry, 292(1):381-393.
View ArticleSantschi, U.P. and Hara, O. P.(1986). Radionuclide cycling in natural waters relevance of scavenging kinetics in sediments and water interactions p 183 springer Berlin.
Seaborg, G.T., Loveland, W. and Morrissey, D. (2006). Modern nuclear chemistry. Wiley interscience p. 57
Shetty, T. and Narayana, A. (2010). Variation of radiation level and radionuclides enrichment in high background area. Journal of Environmental Radioactivity, 101:1043-1047. PMid:20833457
View Article PubMed/NCBIStabin, M. (2007). Radiation protection and dosimetry;An introduction to health physics.
Umar, A.M., Onimisi, M.Y. and Jonah, S.A. (2012). "Baseline measurement of natural radioactivity in soil, vegetation and water in the industrial district of the federal capital territory (FCT) Abuja Nigeria". British Journal of Applied Science and Technology, 2(3):266-274.
View ArticleUNSCEAR (2000). Sources and effects of ionizing radiation United Nations scientific committee on the effects of atomic radiation (New York, USA: United Nations Publication).
Walkley A. and I. A. Black (1934). An examination of Degtjareff method for determining organic carbon in soils: Effects of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 63: 251-263.
View Article
